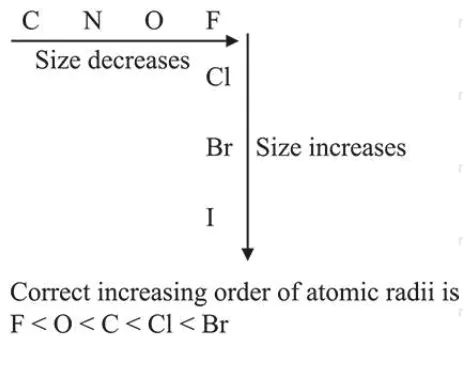
Question: The increasing order of the atomic radii of the follo elements is:
(a) $\mathrm{C}$ (b) $\mathrm{O}$
(c) $\mathrm{F}$ (d) $\mathrm{Cl}$
(e) $\mathrm{Br}$
(b) $<(\mathrm{c})<(\mathrm{d})<(\mathrm{a})<(\mathrm{e})$
$(\mathrm{d})<(\mathrm{c})<(\mathrm{b})<(\mathrm{a})<(\mathrm{e})$
$(\mathrm{c})<(\mathrm{b})<(\mathrm{a})<(\mathrm{d})<(\mathrm{e})$
(a) $<(\mathrm{b})<(\mathrm{c})<(\mathrm{d})<(\mathrm{e})$
Correct Option: , 3
Solution: