Question:
The one that can exhibit highest paramagnetic behaviour among the following is :-
$\mathrm{gly}=\mathrm{glycinato} ; \mathrm{bpy}=2,2^{\prime}-$ bipyridine
Correct Option: , 3
Solution:
$\left[\mathrm{Co}(\mathrm{OX})_{2}(\mathrm{OH})_{2}\right]^{-} \quad \Delta_{0}>\mathrm{P} \quad[\mathrm{S} . \mathrm{F} . \mathrm{L}]$
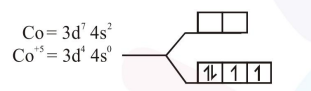
It has highest number of unpaired e-s. so it is most paramagnetic.
