Question:
The oxidation states of iron atoms in compounds (A), (B) and (C), respectively, are $x, y$ and $z$. The sum of $x, y$ and $z$ is
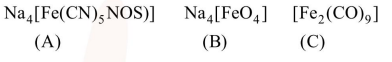
Solution:
(A) $\mathrm{Na}_{4}\left[\mathrm{Fe}(\mathrm{CN})_{5}(\mathrm{NOS})\right]$
$(+1) 4+x+(-1) 5+(-1) 1=0$
$x=+2$
(B) $\mathrm{Na}_{4}\left[\mathrm{Fe} \mathrm{O}_{4}\right]$
$(+1) 4+y+(-2) 4=0$
$y=+4$
(C) $\left[\mathrm{Fe}_{2}^{\mathrm{z}}(\mathrm{CO})_{9}\right]$
$2 z+0 \times 9=0$
$Z=0$
so $(x+y+z)=+2+4+0$
$=6$
