Question: The oxoacid of sulphur that does not contain bond between sulphur atoms is :
$\mathrm{H}_{2} \mathrm{~S}_{4} \mathrm{O}_{6}$
$\mathrm{H}_{2} \mathrm{~S}_{2} \mathrm{O}_{7}$
$\mathrm{H}_{2} \mathrm{~S}_{2} \mathrm{O}_{3}$
$\mathrm{H}_{2} \mathrm{~S}_{2} \mathrm{O}_{4}$
Correct Option: , 2
Solution:
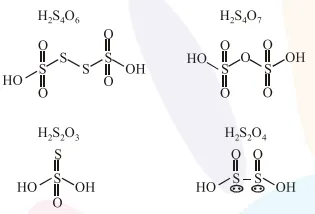
$\mathrm{H}_{2} \mathrm{~S}_{2} \mathrm{O}_{7}$ does not contain bond between sulphur atoms.