Question: The pair in which both the species have the same magnetic moment (spin only) is :
$\left[\mathrm{Mn}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+}$ and $\left[\mathrm{Cr}\left(\mathrm{H}_{2} \mathrm{O}\right)\right]^{2+}$
$\left[\mathrm{Cr}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+}$ and $\left[\mathrm{CoCl}_{4}\right]^{2-}$
$\left[\mathrm{Cr}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+}$ and $\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+}$
$\left[\mathrm{Co}(\mathrm{OH})_{4}\right]^{2-}$ and $\left[\mathrm{Fe}\left(\mathrm{NH}_{3}\right)_{6}\right]^{2+}$
Correct Option: , 3
Solution:
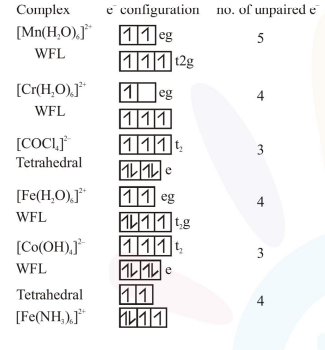
Thus complex $\left[\mathrm{Cr}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+}$ and $\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+}$ have same no. of unpaired $\mathrm{e}^{-}$and hence same magnetic moment (spin only).

