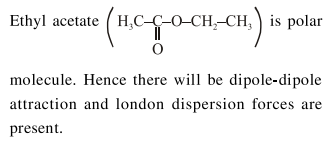
Question: The predominant intermolecular forces present in ethyl acetate, a liquid, are :
hydrogen bonding and London dispersion
Dipole-dipole and hydrogen bonding
London dispersion and dipole-dipole
London dispersion, dipole-dipole and hydrogen bonding
Correct Option: , 3
Solution: