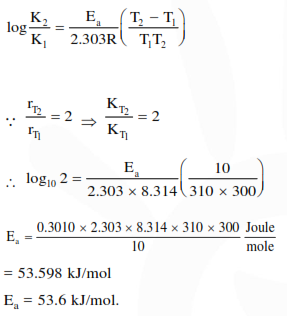Question:
The rate of a reaction doubles when its temperature changes from $300 \mathrm{~K}$ to $310 \mathrm{~K}$. Activation energy of such a reaction will be
$\left(\mathrm{R}=8.314 \mathrm{JK}^{-1} \mathrm{~mol}^{-1}\right.$ and $\left.\log 2=0.301\right)$
Correct Option: 1
Solution: