The variation of equilibrium constant with temperature is given below:
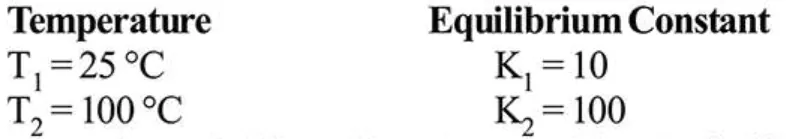
The values of $\Delta \mathrm{H}^{\circ}, \Delta \mathrm{G}^{\circ}$ at $\mathrm{T}_{1}$ and $\Delta \mathrm{G}^{\circ}$ at $\mathrm{T}_{2}$ (in $\mathrm{kj} \mathrm{mol}^{-1}$ ) respectively, are close to
[use $\mathrm{R}=8.314 \mathrm{~J} \mathrm{~K}^{-1} \mathrm{~mol}^{-1}$ ]
Correct Option: , 3
$\Delta \mathrm{G}^{\circ}=-\mathrm{RT} \ln \mathrm{K}, \mathrm{T}_{1}=25^{\circ} \mathrm{C}, \mathrm{K}_{1}=10$
$\Delta \mathrm{G}^{\circ}$ at $\mathrm{T}_{1}=-8.314 \times 298 \times 2.303 \times \log 10=-5.71 \mathrm{~kJ} / \mathrm{mol}$
$\Delta \mathrm{G}^{\circ}$ at $\mathrm{T}_{2}=-8.314 \times 298 \times 373 \times 2.303 \times \log (100)$
$=-14.29 \mathrm{~kJ} / \mathrm{mol}$
$\Delta \mathrm{G}^{\circ}=\Delta \mathrm{H}^{\circ}-\mathrm{T} \Delta \mathrm{S}^{\circ}$
$\Rightarrow-5.71=\Delta \mathrm{H}^{\circ}-298\left(\Delta \mathrm{S}^{\circ}\right)$
$\Rightarrow-14.29=\Delta \mathrm{H}^{\circ}-373\left(\Delta \mathrm{S}^{\circ}\right)$
$\Delta \mathrm{H}^{\circ}=28.4 \mathrm{~kJ} / \mathrm{mol}$
