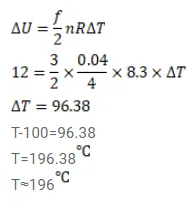Question:
Use $\mathrm{R}=8.3 \mathrm{~J} / \mathrm{mol}-\mathrm{K}$ wherever required.
$0.040 \mathrm{~g}$ of He is kept in a closed container initially at $100.0^{\circ} \mathrm{C}$. The container is now heated. Neglecting the expansion of the container, calculate the temperature at which the internal energy is increased by $12 \mathrm{~J}$.
Solution: