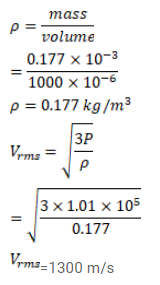Question:
Use $\mathrm{R}=8.3 \mathrm{~J} / \mathrm{mol}-\mathrm{K}$ wherever required.
A sample of $0.177 \mathrm{~g}$ of an ideal gas occupies $1000 \mathrm{~cm}^{3}$ at STP. Calculate the rms speed of the gas molecules.
Solution: