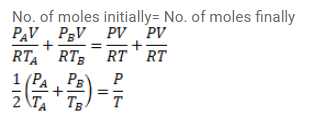Question:
Use $\mathrm{R}=8.3 \mathrm{~J} / \mathrm{mol}-\mathrm{K}$ wherever required.
Figure shows two vessels $\mathrm{A}$ and $\mathrm{B}$ with rigid walls containing ideal gases. The pressure, temperature and the volume are $\rho_{A}, T_{A}, V$ in the vessel $\mathrm{A}$ and $\rho_{B}, T_{B}, V$ in the vessel B. The vessels are now connected through a small tube. Show that the pressure $p$ and the temperature $T$ satisfy
$\frac{p}{T}=\frac{1}{2}\left(\frac{p_{A}}{T_{A}}+\frac{\rho_{B}}{T_{B}}\right)$
When equilibrium is achieved.
Solution: