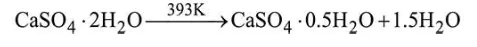Question: When gypsum is heated to $393 \mathrm{~K}$, it forms:
Anhydrous $\mathrm{CaSO}_{4}$
$\mathrm{CaSO}_{4} \cdot 5 \mathrm{H}_{2} \mathrm{O}$
$\mathrm{CaSO}_{4} \cdot 0.5 \mathrm{H}_{2} \mathrm{O}$
Dead burnt plaster
Correct Option: , 3
Solution:
Gypsum on heating to $393 \mathrm{~K}$ forms plaster of Paris.