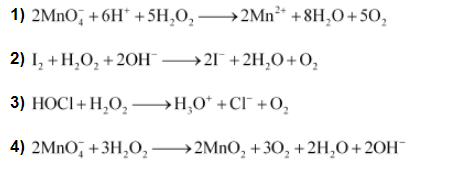Question:
Write chemical reactions to justify that hydrogen peroxide can function as an oxidizing as well as reducing agent.
Solution:
Hydrogen peroxide, H2O2 acts as an oxidizing as well as a reducing agent in both acidic and alkaline media.
Reactions involving oxidizing actions are:
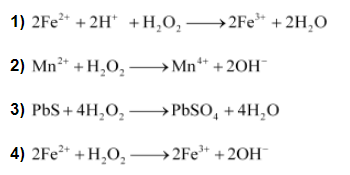
Reactions involving reduction actions are: