JEE Main Previous Year Papers Questions of Chemistry With Solutions are available at eSaral.
Simulator
Previous Years AIEEE/JEE Mains Questions
Q. The alkene that exhibits geometrical isomerism is :-
(1) 2–butene
(2) 2–methyl–2–butene
(3) Propene
(4) 2–methyl propene
[AIEEE-2009]
Ans. (1)
Q. The number of stereoisomers possible for a compound of the molecular formula
CH3–CH=CH–CH(OH)–Me is :-
(1) 4 (2) 6 (3) 3 (4) 2
[AIEEE-2009]
Ans. (1)
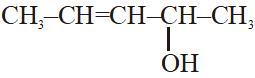 exhibits both geometrical as well as optical isomerism.
Cis – R
Trans – R
Cis – S
Trans – S
exhibits both geometrical as well as optical isomerism.
Cis – R
Trans – R
Cis – S
Trans – S
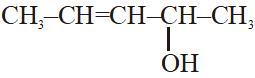 exhibits both geometrical as well as optical isomerism.
Cis – R
Trans – R
Cis – S
Trans – S
exhibits both geometrical as well as optical isomerism.
Cis – R
Trans – R
Cis – S
Trans – S
Q. Out of the following, the alkene that exhibits optical isomerism is :
(1) 2-methyl-2-pentene
(2) 3-methyl-2-pentene
(3) 4-methyl-1-pentene
(4) 3-methyl-1-pentene
[AIEEE-2010]
Ans. (4)
Q. Identify the compound that exhibits tautomerism :-
(1) 2-Pentanone (2) Phenol (3) 2-Butene (4) Lactic acid
[AIEEE-2011]
Ans. (1)
Q. The IUPAC name of the following compounds is :
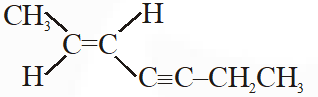 (1) (Z) – 5 – hepten – 3 – yne
(2) (Z) – 2 – hepten – 4 – yne
(3) (E) – 5 – hepten – 3 – yne
(4) (E) – 2 – hepten – 4 – yne
[JEE-Main-2012]
(1) (Z) – 5 – hepten – 3 – yne
(2) (Z) – 2 – hepten – 4 – yne
(3) (E) – 5 – hepten – 3 – yne
(4) (E) – 2 – hepten – 4 – yne
[JEE-Main-2012]
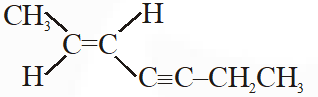 (1) (Z) – 5 – hepten – 3 – yne
(2) (Z) – 2 – hepten – 4 – yne
(3) (E) – 5 – hepten – 3 – yne
(4) (E) – 2 – hepten – 4 – yne
[JEE-Main-2012]
(1) (Z) – 5 – hepten – 3 – yne
(2) (Z) – 2 – hepten – 4 – yne
(3) (E) – 5 – hepten – 3 – yne
(4) (E) – 2 – hepten – 4 – yne
[JEE-Main-2012]
Ans. (4)
Q. Dipole moment is shown by :-
(1) trans-2, 3-dichloro- 2-butene
(2) 1, 2-dichlorobenzene
(3) 1, 4-dichlorobenzene
(4) trans-1, 2-dinitroethene
[JEE-Main 2012]
Ans. (2)
Q. Maleic acid and fumaric acids are :-
(1) Tautomers
(2) Chain isomers
(5) Geometrical isomers
(4) Functional isomers
[JEE-Main 2012]
Ans. (3)
Q. Monocarboxylic acids are functional isomers of :
(1) Esters (2) Amines (3) Ethers (4) Alcohols
[JEE-Main 2013]
Ans. (1)
Monocarboxylic acid and Esters $(-\mathrm{COO}-)$ has same general $(-\mathrm{COOH}) .$
Formula $\left[\mathrm{C}_{\mathrm{n}} \mathrm{H}_{2 \mathrm{n}} \mathrm{O}_{2}\right]$ but different FG, so are called functional group isomer.
Q. Arrange in the correct order of stability (decreasing order) for the following molecules:
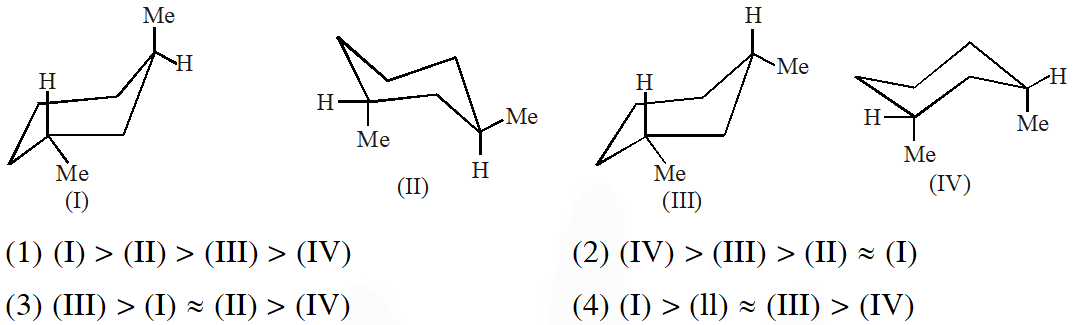 [JEE-Main 2013]
[JEE-Main 2013]
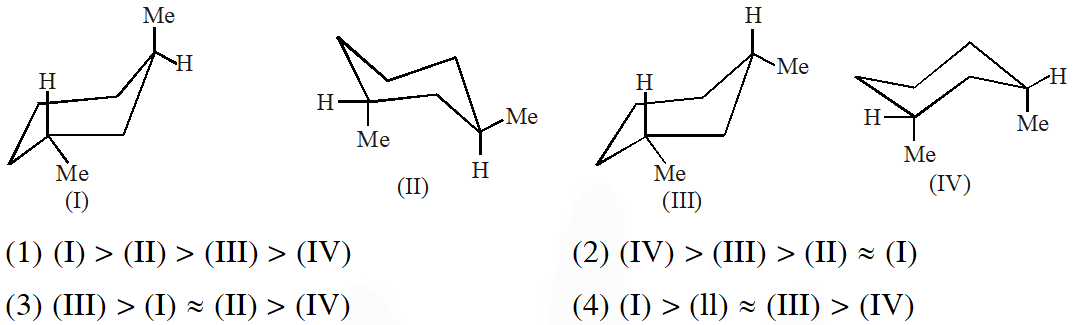 [JEE-Main 2013]
[JEE-Main 2013]
Ans. (3)
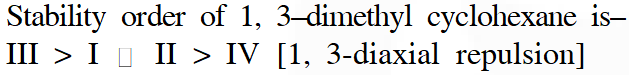
Q. For which of the following molecule significant $\mu \neq 0$
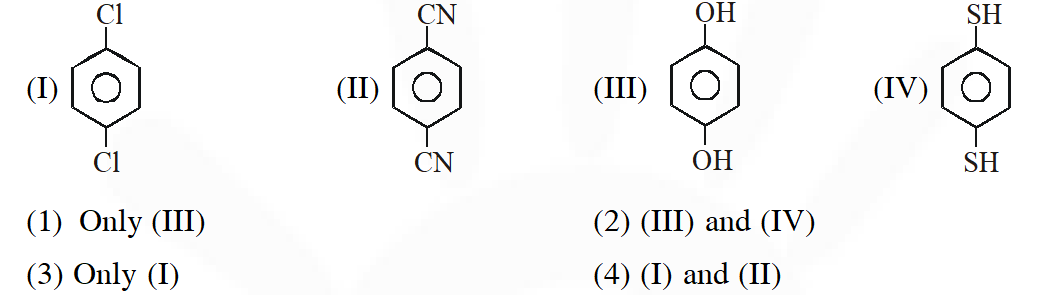 [JEE-Main 2014]
[JEE-Main 2014]
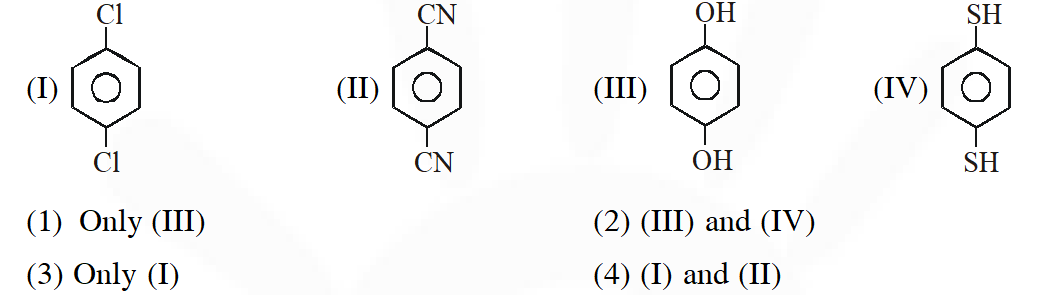 [JEE-Main 2014]
[JEE-Main 2014]
Ans. (2)
Due to presence of $\ell . \mathrm{p}_{(\mathrm{s})}$ on oxygen and sulphur atom which are out of plane hence
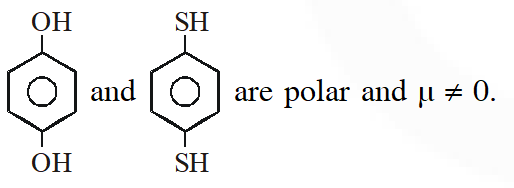
Comments
Venkateswarlu
June 11, 2021, 7 p.m.
Questions are well but answers for the questions also provided then it is very easy to correction and checking
Ritik Ranjan
Feb. 8, 2021, 9:34 p.m.
Dang..These questions don't even require pen.
Guys try out advanced pyqs of isomerism..It's literally worth it..
Answer NEEDED
Nov. 9, 2020, 11:10 a.m.
ANS OF QUESTION 9
........................................................................................................................................................
........................................................................................................................................................
........................................................................................................................................................
........................................................................................................................................................
........................................................................................................................................................
........................................................................................................................................................
sk sukil roy
May 16, 2020, 4:28 p.m.
some what good but very less questions for online very basic but really good to increases your confidence soo
