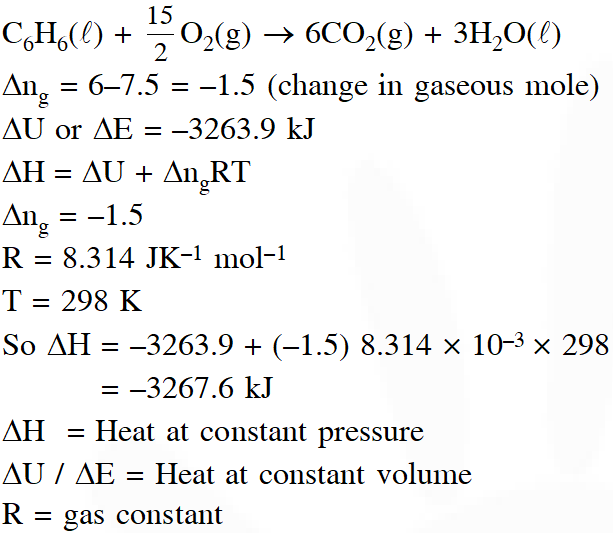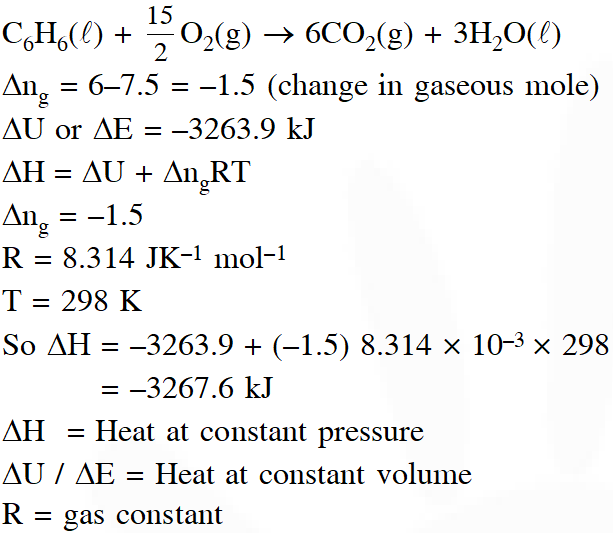JEE Main Previous Year Papers Questions of Chemistry With Solutions are available at eSaral.
Simulator
Previous Years AIEEE/JEE Mains Questions
Q. In a fuel cell methanol is used as fuel and oxygen gas is used as an oxidizer. The reaction is $\mathrm{CH}_{3} \mathrm{OH}(\ell)+\frac{3}{2} \mathrm{O}_{2}(\mathrm{g}) \longrightarrow \mathrm{CO}_{2}(\mathrm{g})+2 \mathrm{H}_{2} \mathrm{O}(\ell)$
At 298 K standard Gibb's energies of formation for $\mathrm{CH}_{3} \mathrm{OH}(\ell), \mathrm{H}_{2} \mathrm{O}(\ell)$ and $\mathrm{CO}_{2}(\mathrm{g})$ are –166.2, –237.2 and –394.4 kJ $\mathrm{mol}^{-1}$ respectively. If standard enthalpy of combustion of methanol is –726 kJ $\mathrm{mol}^{-1}$, efficiency of the fuel cell will be
(1) 90% (2) 97% (3) 80% (4) 87%
[AIEEE-2009]
Ans. (2)
$\mathrm{CH}_{3} \mathrm{OH}(l)+\frac{3}{2} \mathrm{O}_{2}(\mathrm{g}) \rightarrow \mathrm{CO}_{2}+2 \mathrm{D}_{2} \mathrm{O}(l)$
$\Delta \mathrm{G}^{\circ}=\left(2 \Delta \mathrm{G}_{\mathrm{f}}^{\circ}\left[\mathrm{H}_{2} \mathrm{O}(l)+\Delta \mathrm{G}_{\mathrm{f}}^{\circ}\left[\mathrm{CO}_{2}(g)\right]\right)-\left(\Delta \mathrm{G}_{\mathrm{f}}^{\circ}\left[\mathrm{CH}_{3} \mathrm{OH}(l)+\frac{3}{2} \Delta \mathrm{G}_{\mathrm{F}}^{\circ}\left[\mathrm{O}_{2}(g)\right]\right)\right.\right.$
$\Delta \mathrm{G}^{\circ}=(2(-237.2)+(-394.4)-(-166.2+0)$
$\Delta \mathrm{G}^{\mathrm{o}}=-868.8+166.2$
$=-702.6 \mathrm{kJ} / \mathrm{mol}$
Cell efficiency $=\left|\frac{\Delta \mathrm{G}^{\circ}}{\Delta \mathrm{H}^{\circ}}\right| \times 100$
$=\frac{702.6}{726} \times 100=96.77 \approx 97 \%$
Q. On the basis of the following thermochemical data : $\left(\Delta \mathrm{G}_{\mathrm{f}}^{0} \mathrm{H}_{(\mathrm{a}) \mathrm{y}}^{+}=0\right)$
$\mathrm{H}_{2} \mathrm{O}(\ell) \rightarrow \mathrm{H}^{+}(\mathrm{aq})+\mathrm{OH}^{-}(\mathrm{aq}) ; \Delta \mathrm{H}=57.32 \mathrm{kJ}$
$\mathrm{H}_{2}(\mathrm{g})+\frac{1}{2} \mathrm{O}_{2}(\mathrm{g}) \rightarrow \mathrm{H}_{2} \mathrm{O}(\ell) ; \Delta \mathrm{H}=-286.20 \mathrm{kJ}$
The value of enthalpy of formation of $\mathrm{OH}^{-}$ ion at $25^{\circ} \mathrm{C}$ is :-
(1) +228.88 kJ
(2) –343.52 kJ
(3) –22.88 kJ
(4) –228.88 kJ
[AIEEE-2009]
Ans. (4)
$\mathrm{H}_{2} \mathrm{O}(l) \longrightarrow \mathrm{H}^{+}(\mathrm{aq} .)+\mathrm{OH}^{-}(\mathrm{aq} .)$
$57.32=\Delta \mathrm{H}_{\mathrm{f}}^{\mathrm{o}}\left[\mathrm{OH}^{-}(\mathrm{aq})\right]-\Delta \mathrm{H}_{\mathrm{f}}^{\mathrm{o}}\left[\mathrm{H}_{2} \mathrm{O}_{(l)}\right]$
$57.32=\Delta \mathrm{H}_{\mathrm{F}}^{\circ}\left[\mathrm{OH}_{(\mathrm{aq})}^{-}\right]+286.20$
$\Delta \mathrm{H}_{\mathrm{F}}^{\circ}\left[\mathrm{OH}^{-}_{(\mathrm{aq})}\right]=-228.88 \mathrm{kJ}$
Q. The standard enthalphy of formation of $\mathrm{NH}_{3}$ is $-46.0 \mathrm{kJ} \mathrm{mol}^{-1}$ If the enthalpy of formation of H2 from its atoms is –436 kJ $\mathrm{mol}^{-1}$ and that of $\mathrm{N}_{2}$ is $-712 \mathrm{kJ} \mathrm{mol}^{-1}$, the average bond enthalpy of N–H bond in NH3 is
(1) $-1102 \mathrm{kJ} \mathrm{mol}^{-1}$
(2) $-964 \mathrm{kJ} \mathrm{mol}^{-1}$
(3) $+352$ kJ mol $^{-1}$
(4) $+1056 \mathrm{kJ} \mathrm{mol}^{-1}$
[AIEEE-2010]
Ans. (3)
$\frac{1}{2} \mathrm{N}_{2}(\mathrm{g})+\frac{3}{2} \mathrm{H}_{2}(\mathrm{g}) \longrightarrow \mathrm{NH}_{3}(\mathrm{g})$
$-46=\frac{1}{2}(712)+\frac{3}{2}(436)-3(\mathrm{N}-\mathrm{H})$
– 46 = 356 + 654 – 3(N – H)
N – H = 352 kJ / mol
Q. Consider the reaction :
$4 \mathrm{NO}_{2}(\mathrm{g})+\mathrm{O}_{2}(\mathrm{g}) \rightarrow 2 \mathrm{N}_{2} \mathrm{O}_{5}(\mathrm{g}), \Delta_{\mathrm{r}} \mathrm{H}=-111 \mathrm{kJ}$
If $\mathrm{N}_{2} \mathrm{O}_{5}(\mathrm{s})$ is formed instead of $\mathrm{N}_{2} \mathrm{O}_{5}(\mathrm{g})$ in the above reaction, the rH value will be :-
(given, 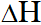 of sublimation for $\mathrm{N}_{2} \mathrm{O}_{5}$ is 54 kJ \mathrm{mol}^{-1})
(1) –165 kJ
(2) +54 kJ
(3) +219 kJ
(4) –219 kJ
[AIEEE-2011]
of sublimation for $\mathrm{N}_{2} \mathrm{O}_{5}$ is 54 kJ \mathrm{mol}^{-1})
(1) –165 kJ
(2) +54 kJ
(3) +219 kJ
(4) –219 kJ
[AIEEE-2011]
Ans. (4)
If $\mathrm{N}_{2} \mathrm{O}_{5}(\mathrm{s})$ is formed instead of $\mathrm{N}_{2} \mathrm{O}_{5}(\mathrm{g})$ then
$\Delta_{1} \mathrm{H}=-111-2(54)$
$\Delta_{\mathrm{r}} \mathrm{H}=-111-108$
$\Delta_{1} \mathrm{H}=-219 \mathrm{kJ} / \mathrm{mol}$
Q. The enthalpy of neutralisation of \mathrm{NH}_{4} \mathrm{OH}with HCl is –51.46 kJ \mathrm{mol}^{-1} and the enthalpy of neutralisation of NaOH with HCl is –55.90 kJ $\mathrm{mol}^{-1}$ The enthalpy of ionisation of $\mathrm{NH}_{4} \mathrm{OH}$ is:
(1) $+107.36 \mathrm{kJ} \mathrm{mol}^{-1}$
(2) $-4.44 \mathrm{kJ} \mathrm{mol}^{-1}$
(3) $-107.36 \mathrm{kJ} \mathrm{mol}^{-1}$
(4) $+4.44 \mathrm{kJ} \mathrm{mo}$
[JEE-mains (online) 2012]
Ans. (4)
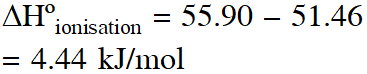
Q. The reaction $\mathrm{X} \rightarrow \mathrm{Y}$ is an exothermic reaction. Activation energy of the reaction for X into Y is 150 kJ $\operatorname{mol}^{-1}$. Enthalpy of reaction is 135 kJ $\mathrm{mol}^{-1}$ . The activation energy for the reverse reaction, $\mathrm{Y} \rightarrow \mathrm{X}$ will be :
(1) 15 kJ $\mathrm{mol}^{-1}$
(2) 285 kJ $\mathrm{mol}^{-1}$
(3) 270 kJ $\mathrm{mol}^{-1}$
(4) 280 kJ $\mathrm{mol}^{-1}$
Ans. (3)
$\Delta \mathrm{H}=\mathrm{E}_{\mathrm{a}(\mathrm{f})}-\mathrm{E}_{\mathrm{a}(\mathrm{b})}$
$-135=150-\mathrm{E}_{\mathrm{a}(\mathrm{b})}$
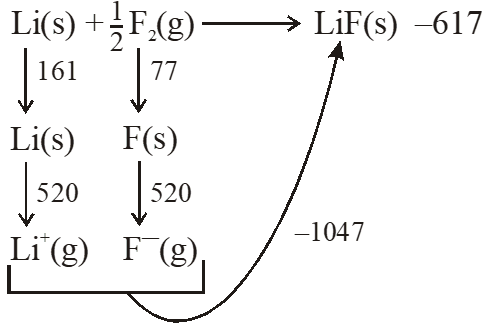 – 617 = 161 + 520 + 77 + x – 1047
x = –328 kJ/mol
– 617 = 161 + 520 + 77 + x – 1047
x = –328 kJ/mol
 – 617 = 161 + 520 + 77 + x – 1047
x = –328 kJ/mol
– 617 = 161 + 520 + 77 + x – 1047
x = –328 kJ/mol
Q. Given
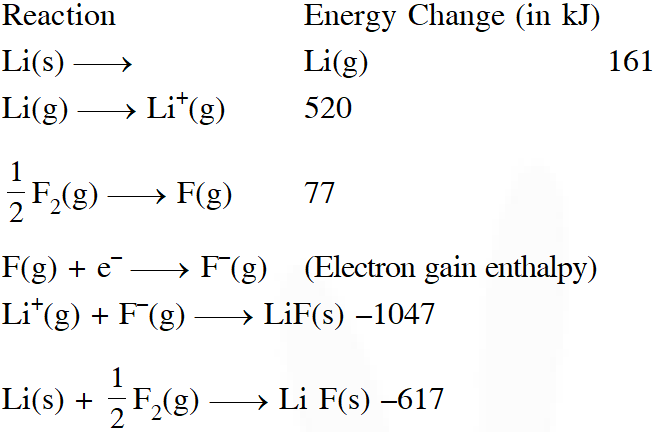 Based on data provided, the value of electron gain enthalpy of fluorine would be :
(1) –300 kJ $\mathrm{mol}^{-1}$
(2) –328 kJ $\mathrm{mol}^{-1}$
(3) –350 kJ $\mathrm{mol}^{-1}$
(4) –228 kJ $\mathrm{mol}^{-1}$
[JEE-mains (online) 2013]
Based on data provided, the value of electron gain enthalpy of fluorine would be :
(1) –300 kJ $\mathrm{mol}^{-1}$
(2) –328 kJ $\mathrm{mol}^{-1}$
(3) –350 kJ $\mathrm{mol}^{-1}$
(4) –228 kJ $\mathrm{mol}^{-1}$
[JEE-mains (online) 2013]
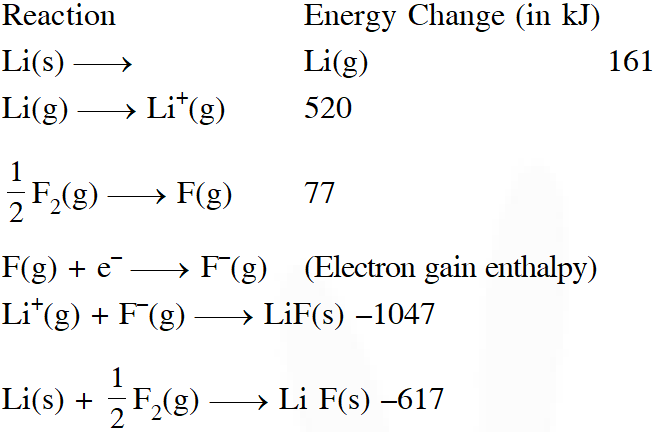 Based on data provided, the value of electron gain enthalpy of fluorine would be :
(1) –300 kJ $\mathrm{mol}^{-1}$
(2) –328 kJ $\mathrm{mol}^{-1}$
(3) –350 kJ $\mathrm{mol}^{-1}$
(4) –228 kJ $\mathrm{mol}^{-1}$
[JEE-mains (online) 2013]
Based on data provided, the value of electron gain enthalpy of fluorine would be :
(1) –300 kJ $\mathrm{mol}^{-1}$
(2) –328 kJ $\mathrm{mol}^{-1}$
(3) –350 kJ $\mathrm{mol}^{-1}$
(4) –228 kJ $\mathrm{mol}^{-1}$
[JEE-mains (online) 2013]
Ans. (2)
Q. Given :
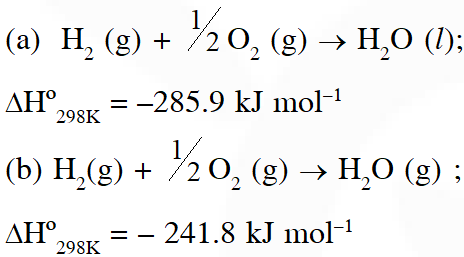 The molar enthalpy of vapourisation of water will be :-
(1) 241. 8 kJ $\mathrm{mol}^{-1}$
(2) 527.7 kJ $\mathrm{mol}^{-1}$
(3) 44.1 kJ $\mathrm{mol}^{-1}$
(4) 22.0 kJ $\mathrm{mol}^{-1}$
[JEE-mains (online) 2013]
The molar enthalpy of vapourisation of water will be :-
(1) 241. 8 kJ $\mathrm{mol}^{-1}$
(2) 527.7 kJ $\mathrm{mol}^{-1}$
(3) 44.1 kJ $\mathrm{mol}^{-1}$
(4) 22.0 kJ $\mathrm{mol}^{-1}$
[JEE-mains (online) 2013]
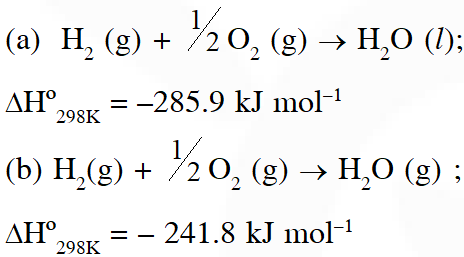 The molar enthalpy of vapourisation of water will be :-
(1) 241. 8 kJ $\mathrm{mol}^{-1}$
(2) 527.7 kJ $\mathrm{mol}^{-1}$
(3) 44.1 kJ $\mathrm{mol}^{-1}$
(4) 22.0 kJ $\mathrm{mol}^{-1}$
[JEE-mains (online) 2013]
The molar enthalpy of vapourisation of water will be :-
(1) 241. 8 kJ $\mathrm{mol}^{-1}$
(2) 527.7 kJ $\mathrm{mol}^{-1}$
(3) 44.1 kJ $\mathrm{mol}^{-1}$
(4) 22.0 kJ $\mathrm{mol}^{-1}$
[JEE-mains (online) 2013]
Ans. (3)
Q. The standard enthalpy of formation $\left(\Delta_{\mathrm{f}} \mathrm{H}_{298}^{\circ}\right)$ for methane, $\mathrm{CH}_{4}$ is– 74.9 kJ $\mathrm{mol}^{-1}$. In order to calculate the average energy given out in the formation of a C–H bond from this it is necessary to know which one of the following?
(1) the dissociation energy of the hydrogen molecule, $\mathrm{H}_{2}$.
(2) the dissociation energy of $\mathrm{H}_{2}$ and enthalpy of sublimation of carbon
(graphite).
(3) the first four ionisation energies of carbon and electron affinity of hydrogen.
(4) the first four ionisation energies of carbon.
[JEE-mains(online) 2014]
Ans. (2)
From formation enthalpy of methane C-H bond enthalpy can be calculated as –
$\mathrm{C}(\mathrm{s})+2 \mathrm{H}_{2}(\mathrm{g}) \longrightarrow \mathrm{CH}_{4}(\mathrm{g})$
$\Delta \mathrm{H}_{\mathrm{f}}^{\circ}\left(\mathrm{CH}_{4}(\mathrm{g})\right)=\Delta \mathrm{H}_{\mathrm{a}}^{\circ}[\mathrm{C}(\mathrm{s})]+2(\mathrm{H}-\mathrm{H})-4(\mathrm{C}-\mathrm{H})$
$(\mathrm{C}-\mathrm{H})=\frac{\Delta \mathrm{H}_{\mathrm{f}}^{\circ}\left(\mathrm{CH}_{4}(\mathrm{g})\right)-\Delta \mathrm{H}_{\mathrm{f}}^{\circ}(\mathrm{C}(\mathrm{s}))-2[\mathrm{H}-\mathrm{H}]}{4}$
So for calculating (C-H) bond we will required data of H- H bond enthalpy and sublimation enthalpy of carbon solid.
Q. For an ideal Solution of two components A and B, which of the following is true ?
(1) $\Delta \mathrm{H}_{\text {mixing }}<0$ (zero)
(2) A – A, B – B and A – B interactions are identical
(3) A – B interaction is stronger than A – A and B – B interactions
(4) $\Delta \mathrm{H}_{\text {mixing }}>0$ (zero)
[JEE-mains (online) 2014]
Ans. (1)
Q. For complete combustion of ethanol, $\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}(\ell)+3 \mathrm{O}_{2}(\mathrm{g}) \rightarrow 2 \mathrm{CO}_{2}(\mathrm{g})+3 \mathrm{H}_{2} \mathrm{O}(\ell)$the amount of heat produced as measured in bomb calorimeter, is 1364.47 kJ $\mathrm{mol}^{-1}$ at $25^{\circ} \mathrm{C}$. Assuming ideality the Enthalpy of combustion, $\Delta_{\mathrm{C}} \mathrm{H}$, for the raction will be :-
$\left(\mathrm{R}=8.314 \mathrm{kJ} \mathrm{mol}^{-1}\right)$
(1) $-1460.50 \mathrm{kj} \mathrm{mol}^{-1}$
(2) $-1350.50 \mathrm{kJ} \mathrm{mol}^{-1}$
(3) $-1366.95 \mathrm{kJ} \mathrm{mol}^{-1}$
$(4)-1361.95 \mathrm{kJ} \mathrm{mol}^{-1}$
[JEE-mains(offline)2014]
Ans. (3)
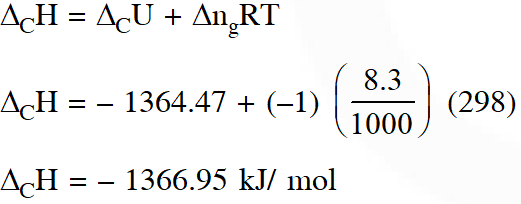
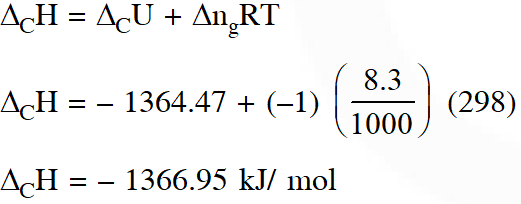
Q. The heats of combustion of carbon and carbon monoxide are – 393.5 and – 285.5 kJ $\operatorname{mol}^{-1}$, respectively. The heat of formation (in kJ) of carbon monoxide per mole is :
(1)– 110
(2) 110.5
(3) 676.5
(4) – 676.5
[JEE-Mains 2016]
Ans. (1)
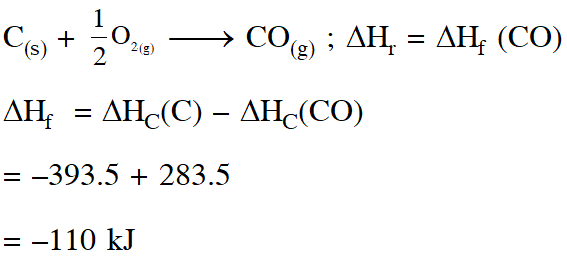
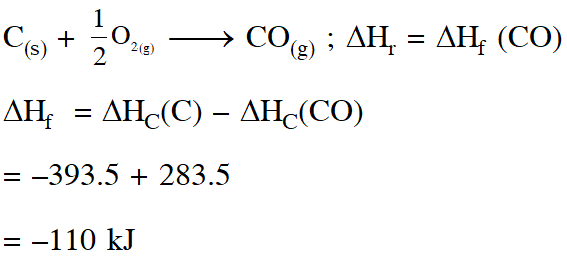
Q. Given
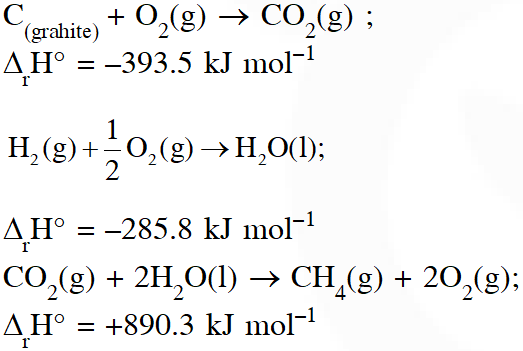
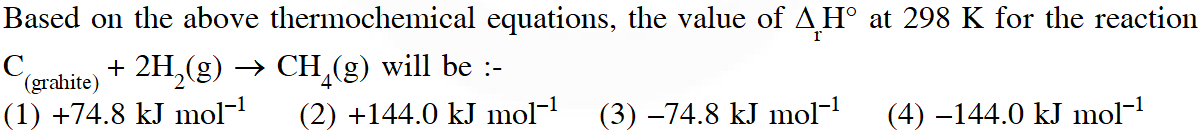 [JEE - Main - 2017]
[JEE - Main - 2017]
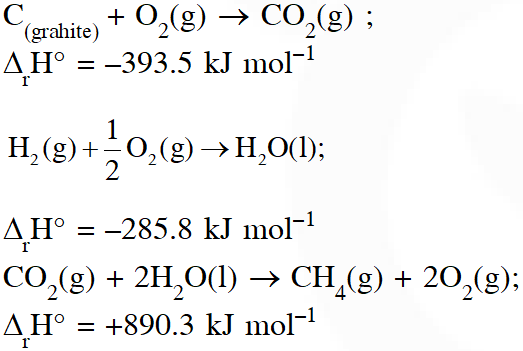
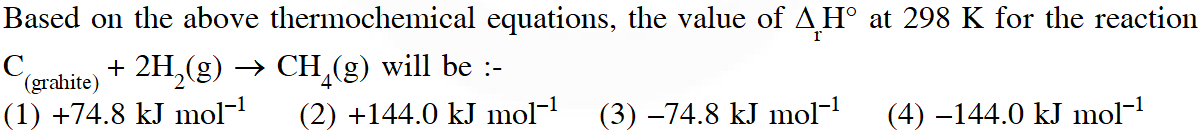 [JEE - Main - 2017]
[JEE - Main - 2017]
Ans. (3)
$\mathrm{CO}_{2}(\mathrm{g})+2 \mathrm{H}_{2} \mathrm{O}(\ell) \rightarrow \mathrm{CH}_{4}(\mathrm{g})+2 \mathrm{O}_{2}(\mathrm{g}) ; \Delta \mathrm{H}^{\circ}=890.3$
$\Delta_{\mathrm{f}} \mathrm{H}^{\circ}-393.5-285.8$
$\Delta_{\mathrm{r}} \mathrm{H}^{\circ}=\sum\left(\Delta_{\mathrm{f}} \mathrm{H}^{\circ}\right)_{\text {protucts }}-\sum\left(\Delta_{\mathrm{f}} \mathrm{H}^{\circ}\right)_{\text {Restatatis }}$
$890.3=\left[1 \times\left(\Delta_{\mathrm{f}} \mathrm{H}^{\circ}\right)_{\mathrm{CH}_{4}}+2 \times 0\right]-[1 \times(-393.5)+2(-285.8)]$
$\left(\Delta_{\mathrm{f}} \mathrm{H}^{\circ}\right)_{\mathrm{CH}_{4}}=890.3-965.1=-74.8 \mathrm{kJ} / \mathrm{mol}$
Q. The combustion of benzene (l) gives $\mathrm{CO}_{2}(\mathrm{g})$ and $\mathrm{H}_{2} \mathrm{O}(\mathrm{l})$. Given that heat of combustion
of benzene at constant volume is –3263.9 kJ $\operatorname{mol}^{-1}$ at $25^{\circ} \mathrm{C}$; heat of combustion (in kJ $\mathrm{mol}^{-1}$) of benzene at constant pressure will be - (R = 8.314 $\mathrm{JK}^{-1}$ $\mathrm{mol}^{-1}$)
(1)–452.46
(2) 3260
(3) –3267.6
(4) 4152.6
[JEE - Main - 2018]
Ans. (3)