Notes of Carbon and Its Compounds Class 10
Class 10.webp)
Notes of Carbon and Its Compounds Class 10- Carbon and its compounds form an integral part of the Class 10 Science curriculum. This topic covers the study of various organic and inorganic compounds of carbon, their properties, and uses in our daily lives. Understanding the basics of carbon and its compounds is crucial for gaining a strong foundation in chemistry, and it also serves as a stepping stone for advanced studies in this field. In this article, we present a comprehensive set of notes on carbon and its compounds for Class 10 students. These notes cover all the essential concepts and topics related to carbon, including its electronic configuration, bonding, organic and inorganic compounds, and their properties. By going through these notes, students can not only clear their concepts but also prepare themselves for their Class 10 board exams. So, let's dive into the world of carbon and its compounds and explore their amazing properties and applications.
Bonding in carbon and covalent bond
In Carbon Bonding, Carbon forms a covalent bond due to its atomic number of 6 and electronic configuration of 2,4. It needs 4 electrons to achieve the inert gas electronic configuration, but it cannot form an ionic bond. Carbon could gain four electrons to form a C4- cation, but the nucleus with six protons would find it difficult to hold on to ten electrons.
The electron dot structure of carbon shows how the covalent bond is formed between two atoms by sharing electrons. Physical properties of organic compounds are determined by the type of covalent bonding that exists between atoms.
Carbon has several allotropes, such as diamond, graphite, and fullerenes, which have different physical properties due to variations in their bonding structures.
Carbon has the option to lose four electrons to form C4+ cations, but it would require a considerable amount of energy to remove them. Therefore, carbon solves this issue by sharing its valence electrons with other carbon atoms or atoms of other elements.
When two atoms share valence electrons to form a bond in a molecule, it is called a Covalent Bond.
Types of Covalent Bond
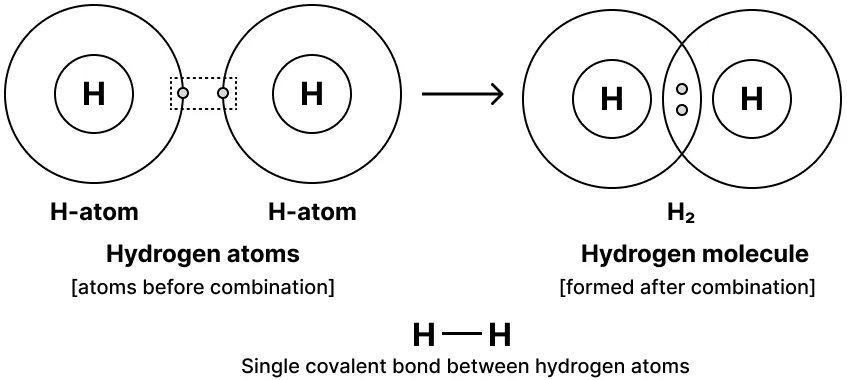
When two atoms share a single pair of electrons in a molecule, it forms a Single Covalent Bond. Examples of molecules with a single covalent bond include F2, Cl2, and H2.
A Double Covalent Bond is formed when two pairs of electrons are shared between two atoms in a molecule. Examples of molecules with double covalent bonds include O2 and CO2.
A Triple Covalent Bond is formed when three pairs of electrons are shared between two atoms in a molecule. An example of a molecule with a triple covalent bond is N2.
The Electron Dot Structure provides a visual representation of the bonding in molecules based on the shared pairs of electrons and octet rule.
Formation of Hydrogen Molecule - Notes of Carbon and Its Compounds Class 10
Atomic number of Hydrogen = 1
Number of valence electrons = 1
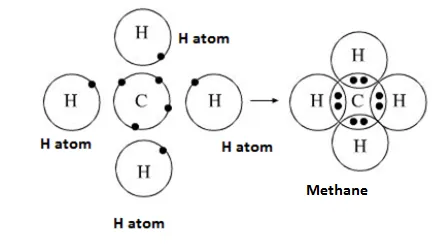
Formation of CH4 Molecule
Atomic number of Carbon = 6 [2, 4]
Number of valence electrons = 4
Atomic number of Hydrogen = 1
Number of valence electrons = 1
Formation of CO2 Molecule - Notes of Carbon and Its Compounds Class 10
Atomic number of Carbon = 6 [2, 4]
Number of valence electrons = 4
Atomic number of Oxygen = 8 [2, 6]
Number of valence electrons = 6
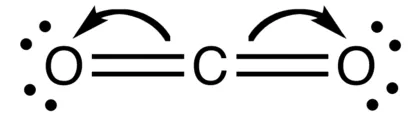
Physical Properties of Organic Compounds - Notes of Carbon and Its Compounds Class 10
- The weak force of attraction between molecules (i.e., intermolecular force of attraction) causes most organic compounds to have a low boiling and melting point.
- Carbon compounds typically do not conduct electricity well because of the absence of free electrons and ions.
| Compounds | M.P. (K) | B.P. (K) |
| Acetic acid (CH3COOH) | 290 | 391 |
| Chloroform (CHCl3) | 209 | 334 |
| Ethanol (CH3CH2OH) | 156 | 351 |
| Methane (CH4) | 90 | 111 |
Allotropes of Carbon - Notes of Carbon and Its Compounds Class 10
The phenomenon in which the element exists in two or more different physical states with similar chemical properties are called Allotropy.
Carbon has Three Main Allotropes
- In Diamond, carbon atoms form three-dimensional structures by bonding with four other carbon atoms. Diamond is the hardest substance and an insulator, commonly used for drilling rocks, cutting, and making jewelry.
- Graphite consists of carbon atoms bonded to three other carbon atoms. It is an excellent conductor of electricity and is used as a lubricant.
- Buckminster Fullerene is an allotrope of carbon consisting of 60 carbon atoms joined together to form spherical molecules. It exists as a dark solid at room temperature.
Versatile Nature of Carbon:
- The versatile nature of carbon, which includes its ability to catenate (form long chains), and its tetravalent nature, is the reason for the existence of a vast number of organic compounds.
- Hydrocarbons are organic compounds that contain only carbon and hydrogen atoms.
- Isomerism refers to the phenomenon where two or more compounds have the same molecular formula but different structural formulas.
- A Homologous Series is a series of organic compounds that have the same functional group and exhibit similar chemical properties.
- Functional Groups are specific groups of atoms that give a molecule its characteristic properties and determine its reactivity.
- The Nomenclature of Functional Groups involves naming organic compounds based on their functional groups and substituents.
(i) Catenation:
- Catenation is the property of an element, primarily the carbon atom, to form long chains, branches, and rings of different sizes through covalent bonds with itself.
- This property is due to several factors, including the small size of the carbon atom and the great strength of the carbon-carbon bond.
- Additionally, carbon has the ability to form stable multiple bonds (double or triple) with itself and with atoms of other elements.
Straight Chain
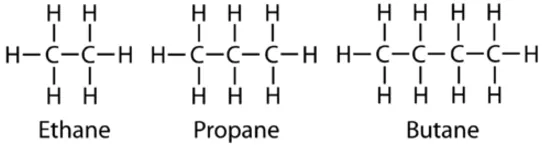
(ii) Tetravalent Nature: Carbon has valency of four. It is capable of bonding with four other atoms of carbon or some other heteroatoms with single covalent bond as well as double or triple bond.
Chloroethane structure
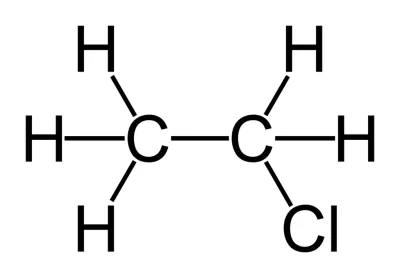
Ethanal
.webp)
Ethanol
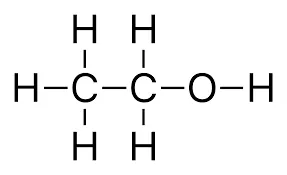
Hydrocarbons: Compounds of carbon and hydrogen are known as hydrocarbons.
For example; Methane (CH4), Ethane (C2H6), Ethene (C2H4), Ethyne (C2H2) etc
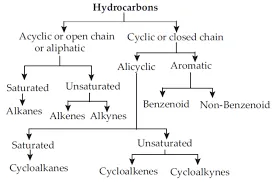
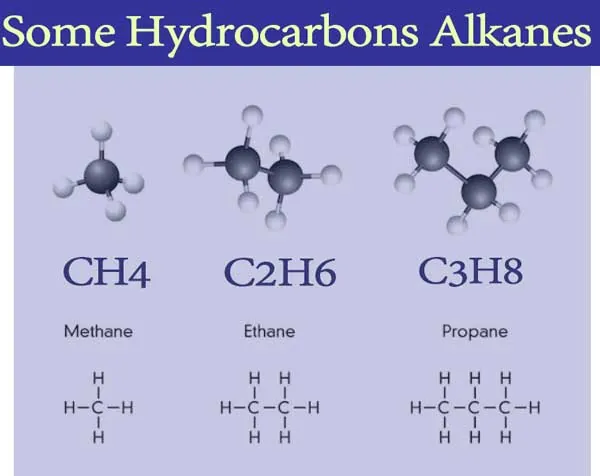
Saturated Hydrocarbon (Alkanes): General formula is CnH2n+2.
n = number of carbon atoms.
In this, the carbon atoms are connected by only a single bond.
For example; Methane (CH4), Ethane (C2H6) etc.
Unsaturated Hydrocarbons:
General formula is CnH2n, where n = number of carbon atoms.In this, the two carbon atoms are connected by double bond.
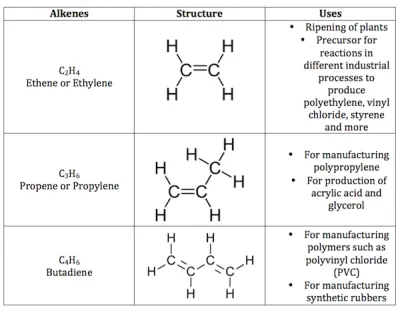
Alkynes: General formula is CnH2n-2, where n = number of carbon atoms. In this, the two carbon atoms are connected by triple bond.
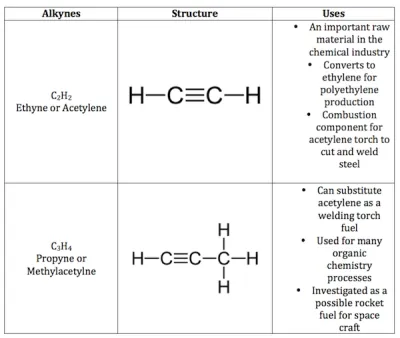
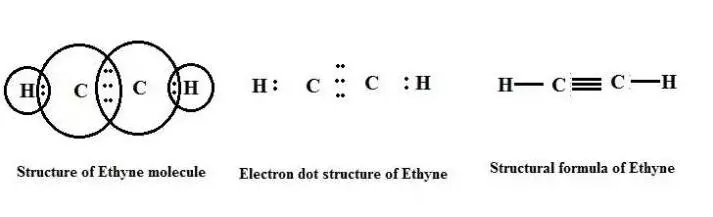
Electron Dot Structure of Hydrocarbons - Notes of Carbon and Its Compounds Class 10
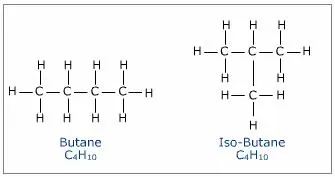
Isomerism:Isomerism is the phenomenon where compounds have the same molecular formula but different structural formulas and properties, and these compounds are known as isomers.
Structural Isomerism: Compounds having the same molecular formula but different structures are called Structural isomers. Example: Isomers of butane (C4H10)
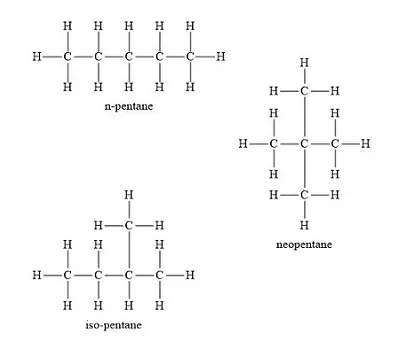
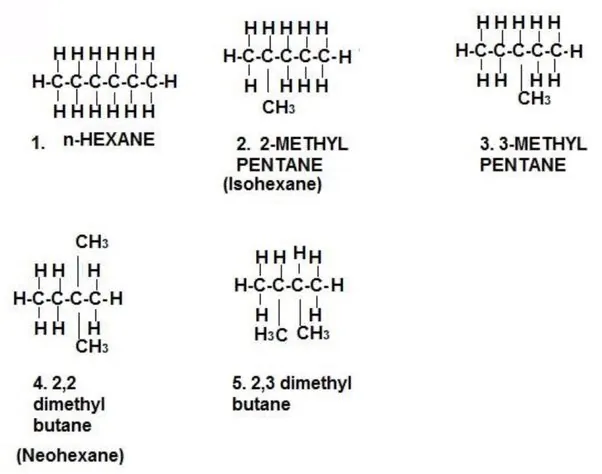 Homologous Series: A homologous series is a series of organic compounds that have the same functional group and exhibit similar chemical properties, with successive members differing by a CH2 unit or 14 mass units.
Homologous Series: A homologous series is a series of organic compounds that have the same functional group and exhibit similar chemical properties, with successive members differing by a CH2 unit or 14 mass units. 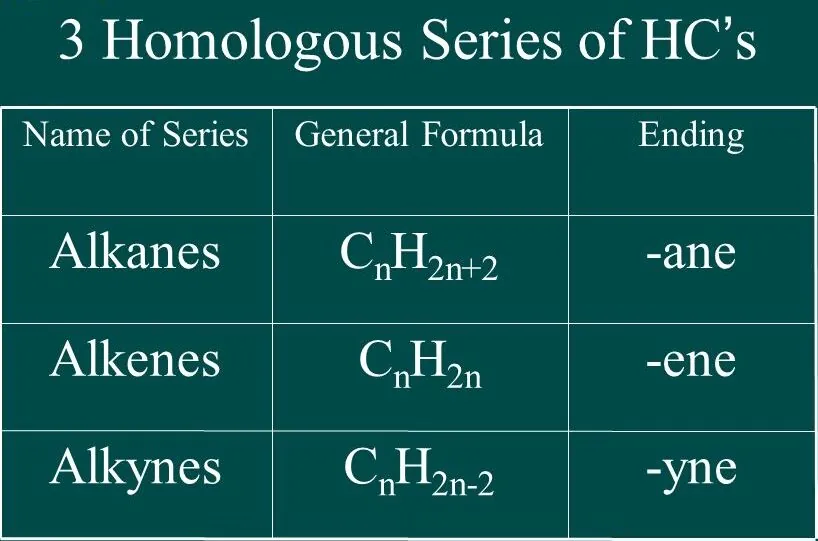
Characteristic of Homologous Series
- The successive members in homologous series differ by CH2 unit or 14 mass unit.
- Members of given homologous series have the same functional group.
- All the members of homologous series shows similar chemical properties.
Functional Group
An atom or group of atoms present in a molecule which largely determines its chemical properties are called Functional Group.
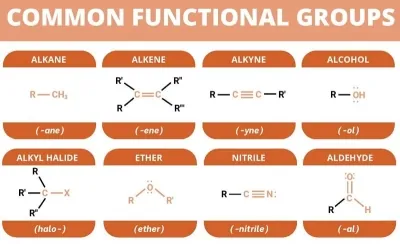
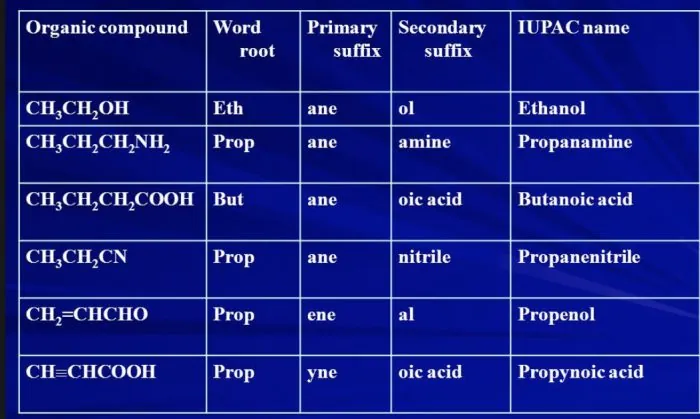
Nomenclature of Organic Compounds
To systematize the nomenclature of organic compounds, certain rules have been given by the International Union of Pure and Applied Chemistry (IUPAC), as it is difficult to remember millions of compounds by their individual common name.
| S. No | Number of Carbon Atoms | Word Root (-) (Suffix) | Single bond |
| 1. | One carbon atoms (1-C) | Meth | + ane |
| 2. | Two carbon atoms (2-C) | Eth | + ane |
| 3. | Three carbon atoms (3-C) | Prop | + ane |
| 4. | Four carbon atoms (4-C) | But | + ane |
| 5. | Five carbon atoms (5-C) | Pent | + ane |
| 6. | Six carbon atoms (6-C) | Hex |
+ ane
|
Identify the functional group
| S. No. | Functional Group | Prefix | Suffix |
| 1. | Double bond (=) | — | ene |
| 2. | Triple bond (≡) | — | yne |
| 3. | Chlorine (—Cl) | Chloro | — |
| 4. | Bromine (—Br) | Bromo | — |
| 5. | Alcohol (-OH) | — | ol |
| 6. | Aldehyde (-CHO) | — | al |
| 7. | Ketone (-CO-) | — | one |
| 8. | Carboxylic acid (-COOH) | — |
oic acid |
Name the Compounds By Following Order
Prefix + Word Root + Suffix
Chemical Properties of Carbon Compounds
1. Combustion: When carbon compounds are completely combusted in the air, they give carbon dioxide, water, heat, and light, as shown in the following equation:
CH3CH2OH(l) + O2(g) → CO2(g) + H2O(l) + heat and light
Carbon dioxide and heat are produced when carbon burns in air or oxygen according to the following equation: C(s) + O2(g) → CO2(g) + Heat and light
When saturated hydrocarbons burn with a sufficient supply of air or oxygen, carbon dioxide, water, heat, and light are produced. The following equation represents the combustion of methane (CH4): CH4(g) + 2O2(g) → CO2(g) + 2H2O(l) + Heat and light
However, when saturated hydrocarbons burn in the presence of a limited supply of air, a sooty flame is produced due to incomplete combustion.
Unsaturated hydrocarbons, on the other hand, burn with a yellow smoky flame.
Gas and kerosene stoves used at home have an inlet for air to ensure complete combustion and a clean blue flame.
Coal and petroleum contain small amounts of nitrogen and sulfur, which, when burned, produce carbon dioxide along with oxides of nitrogen and sulfur. These oxides are major pollutants.
2. Oxidation: The oxidation of ethanol in the presence of oxidizing agents can be represented by the following equation:
CH3CH2OH + [O] → CH3COOH + H2O
where [O] represents the oxidizing agent.
Oxidizing Agent : Some substances are capable of adding oxygen to others, are known as Oxidising Agent.
Example: Alkaline KMnO4 (or KMnO4—KOH)
Acidified K2Cr2O7 (or K2Cr2O7—H2SO4)
KMnO4 – Potassium permanganate
K2Cr2O7 – Potassium dichromate
3. Addition Reaction: Addition of dihydrogen with unsaturated hydrocarbon in the presence of catalysts such as nickel or platinum or palladium are known as Hydrogenation (addition) reaction.
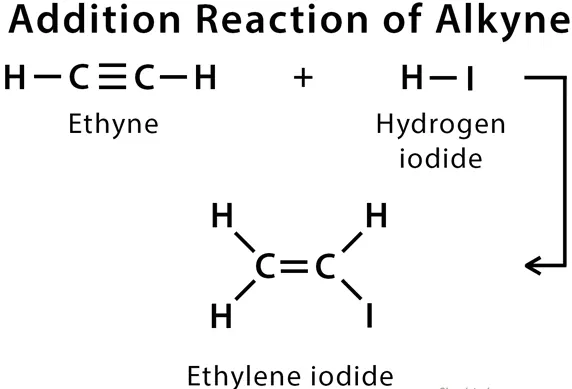
Catalyst: Substances that cause a reaction to occur or proceeds to different rate without consuming in it are called a catalyst. For example; Ni, Pt, Pd, etc.
The process of hydrogenation of oil can be represented by the following equation, where vegetable oil (C18H34O2) reacts with hydrogen gas (H2) in the presence of a catalyst (such as Ni, Pt, Pd, etc.) to form solid fat (vegetable ghee) and water:
C18H34O2 + H2 → C18H36O2 + H2O
Vegetable fats are saturated fats which are harmful for health.
Vegetable oil containing unsaturated fatty acids are good for health.
4. Substitution Reaction:
In an organic substitution reaction, one or more hydrogen atoms in a molecule are replaced by another atom or group. An example of a substitution reaction is the reaction between methane (CH4) and chlorine gas (Cl2) to form chloromethane (CH3Cl) and hydrogen chloride (HCl):
CH4 + Cl2 → CH3Cl + HCl
In this reaction, one of the hydrogen atoms in methane is replaced by a chlorine atom to form chloromethane.
The equation for a general substitution reaction involving an organic molecule (R-H) and a substituting species (X) can be written as:
R-H + X → R-X + H-X
In this equation, X represents the atom or group of atoms that is replacing one or more hydrogen atoms in the organic molecule (R-H), and H-X represents the product formed by the reaction of the substituting species with the hydrogen atom(s) removed from the organic molecule.
Some Important Carbon Compounds :
Ethanol (CH3CH2—OH): Commonly known as Ethyl Alcohol.
Physical Properties
- It is colourless, inflammable liquid.
- It is miscible with water in all proportions.
- It has no effect on the litmus paper.
Chemical Properties
Reaction with sodium
2 Na + 2 C2H5OH → 2 C2H5ONa + H2
In this reaction, sodium (Na) reacts with ethanol (C2H5OH) to form sodium ethoxide (C2H5ONa) and hydrogen gas (H2).
Dehydrating agent: The substance that removes water from ethanol (alcohols) is known as a dehydrating agent. An example of a dehydrating agent is concentrated sulfuric acid (H2SO4):
C2H5OH + H2SO4 → C2H5HSO4 + H2O
In this reaction, concentrated sulfuric acid removes a molecule of water (H2O) from ethanol (C2H5OH) to form ethyl hydrogen sulfate (C2H5HSO4).
Dehydrating agents like concentrated sulfuric acid have several uses, including as a solvent, antiseptic (in tincture iodine), and anti-freeze in automobiles.
Ethanoic acid (CH3COOH), commonly known as acetic acid, is a weak organic acid. A 5-8% solution of ethanoic acid in water is known as vinegar. The melting point of pure ethanoic acid is 290 K, which is why it often freezes in cold climates, hence its name, glacial acetic acid.
Some physical properties of ethanoic acid are:
- It is a colourless, pungent-smelling liquid.
- It is miscible with water in all proportions.
- It turns blue litmus to red.
Chemical Properties
(i) Esterification Reaction: The esterification reaction is a reaction between an alcohol and a carboxylic acid in the presence of a catalyst, usually concentrated sulfuric acid (H2SO4). For example, the reaction between ethanoic acid and ethanol can be written as:
CH3COOH + C2H5OH ⇌ CH3COOC2H5 + H2O
In this reaction, ethanoic acid reacts with ethanol in the presence of concentrated sulfuric acid as a catalyst to form ethyl acetate and water.
The sweet-smelling substance formed in this reaction is an ester, which is a class of organic compounds that are commonly used as fragrances and flavors. Esterification reactions are widely used in the production of perfumes, cosmetics, and food flavorings.
Saponification Reaction: Saponification reaction is a reaction between an ester and a strong base, usually sodium hydroxide (NaOH), which results in the formation of an alcohol and the sodium salt of a carboxylic acid (soap). The equation for the saponification reaction is as follows:
RCOOR' + NaOH → R'OH + NaRCO2
In this equation, R and R' represent the organic groups attached to the carbonyl (C=O) group in the ester (RCOOR'). The reaction between an ester such as ethyl acetate (CH3COOCH2CH3) and sodium hydroxide can be written as:
CH3COOCH2CH3 + NaOH → CH3CH2OH + NaCH3COO
In this reaction, ethyl acetate reacts with sodium hydroxide to form ethanol (CH3CH2OH) and sodium acetate (NaCH3COO), which is the sodium salt of acetic acid.
Saponification reactions have many practical applications, including the production of soaps and detergents, and the preparation of pharmaceuticals and cosmetics.
(ii) Reaction with Carbonates and Hydrogen Carbonates: When ethanoic acid reacts with sodium carbonate (Na2CO3) or sodium hydrogen carbonate (NaHCO3), a salt, carbon dioxide, and water are produced. The balanced chemical equations for these reactions are as follows:
CH3COOH + Na2CO3 → 2NaCH3COO + CO2 + H2O
CH3COOH + NaHCO3 → NaCH3COO + CO2 + H2O
In these equations, ethanoic acid (CH3COOH) reacts with either sodium carbonate or sodium hydrogen carbonate to form sodium acetate (NaCH3COO), carbon dioxide (CO2), and water (H2O).
These reactions are commonly used in the preparation of effervescent tablets and antacids, where the carbon dioxide produced helps to relieve symptoms of indigestion and heartburn.
- Used as vinegar.
- Used as raw material for the preparation of acetyl chloride and esters.
Soap: Sodium or potassium salts of long chain fatty acids is called Soap.
General formula: RCOO–Na+
Detergent: Ammonium and sulphonate salts of long chain fatty acids are called Detergent.
Example: CH3—(CH2)11—C6H4—SO3Na.
Hard and Soft Water: Water that does not produce lather with soap readily is referred to as hard water, while water that produces lather with soap is known as soft water. The hardness of water is caused by the presence of calcium and magnesium salts, such as bicarbonates, chlorides, and sulfates.
The reaction between calcium or magnesium ions in hard water and the soap molecule results in the formation of insoluble calcium or magnesium salts of fatty acids, which reduces the effectiveness of soap. The equation for the reaction between soap and calcium ions in hard water can be written as:
2RCOO^-Na+ + Ca2+ → (RCOO)2Ca↓ + 2Na+
In this equation, RCOO^- represents the soap molecule, Na+ represents the sodium ion, and Ca2+ represents the calcium ion. The symbol ↓ indicates the formation of a precipitate.
To remove the hardness of water, it can be treated with certain chemicals, such as washing soda (Na2CO3) or lime (Ca(OH)2). These chemicals react with calcium and magnesium ions to form insoluble calcium and magnesium salts that can be removed by filtration. The equations for the reactions between washing soda and calcium and magnesium ions can be written as:
Na2CO3 + Ca2+ → CaCO3↓ + 2Na+
Na2CO3 + Mg2+ → MgCO3↓ + 2Na+
In these equations, Na2CO3 represents washing soda, Ca2+ represents the calcium ion, Mg2+ represents the magnesium ion, and ↓ indicates the formation of a precipitate.
Difference between soaps and detergents
| Property | Soap | Detergent |
|---|---|---|
| Chemical composition | Made from natural ingredients like fats and oils | Made from synthetic chemicals |
| Effectiveness | Less effective in hard water | More effective in hard water |
| Lather | Produces less lather | Produces more lather |
| Environment | Biodegradable | Non-biodegradable |
| Cost | Cheaper than detergents | More expensive than soap |
| Stains | Less effective on oil-based stains | More effective on oil-based stains |
| Fabric care | Can leave a residue on clothes, causing them to look dull | Doesn't leave residue on clothes, keeping them looking bright |
Overall, while soap is a more natural and biodegradable option, it can be less effective in hard water and less efficient at removing oil-based stains. On the other hand, detergents are more synthetic and non-biodegradable, but they are more effective in hard water and at removing stains.
Advantage of Detergents: Detergent has a main advantage over soap in that it can be used for washing in hard water without forming a curdy white precipitate called scum, which is formed when hard water reacts with soap.
The reaction between soap and calcium or magnesium ions in hard water results in the formation of insoluble calcium or magnesium salts of fatty acids, which are visible as scum. The equation for the reaction between soap and calcium ions in hard water is:
2RCOO^-Na+ + Ca2+ → (RCOO)2Ca↓ + 2Na+
In this equation, RCOO^- represents the soap molecule, Na+ represents the sodium ion, and Ca2+ represents the calcium ion. The symbol ↓ indicates the formation of a precipitate.
On the other hand, detergents are made from synthetic chemicals and do not react with calcium and magnesium ions in hard water, making them effective in both hard and soft water. This property of detergents is due to their chemical structure, which contains both hydrophilic (water-loving) and hydrophobic (water-repelling) parts.
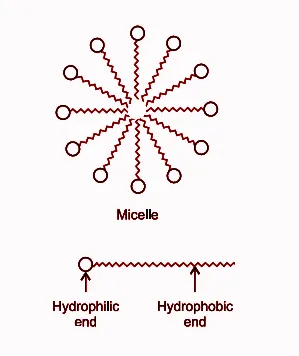
Cleansing Action of Soaps and Detergents :
Both soaps and detergents consist of two parts: a long hydrocarbon chain (HC) and a short ionic head (IH). The HC is hydrophobic (repels water) while the IH is hydrophilic (attracts water).
The soap molecule's HC part attaches itself to the oily (dirt) droplet, and the IH part aligns itself with the water to form micelles, which are spherical structures. This can be expressed as:
HC + Oily droplet ⇌ HC-Oily droplet IH + Water ⇌ IH-Water
The soap micelles aid in dissolving dirt in water and washing our clothes. This can be represented as:
HC-Oily droplet + IH-Water → Micelle Micelle + Dirt → Micelle-Dirt complex Micelle-Dirt complex + Water → Solution
Covalent Bond: A chemical bond formed between two atoms by sharing of valence electrons between two atoms so that each atom acquires the stable electronic configuration of the nearest noble gas
Covalency: The number of electrons contributed by each atom for sharing.
Carbon always forms a covalent bond: The atomic number of carbon is 6, which implies that it has a configuration of K-2, L-4. In order to attain a noble gas configuration and become stable, it needs to either lose or gain 4 electrons. However, carbon faces difficulty in doing so due to the following reasons:
Firstly, it cannot gain 4 electrons to form a C4- ion with a Neon gas (2,8) configuration because the anion would be highly unstable. This is because a large amount of energy is required to overcome the repulsive forces between the four electrons being added and the six electrons already present in the carbon atom.
Secondly, it cannot lose 4 electrons to form a C4+ ion with a Helium gas (2) configuration because the resulting cation would be highly unstable. This is because a large amount of energy is required to remove four electrons from the carbon atom.
Conclusion
In conclusion to Notes of Carbon and Its Compounds Class 10, the study of Carbon and its compounds is an essential topic for students in Class 10. The Notes of Carbon and Its Compounds Class 10 cover a vast range of concepts, from the properties and characteristics of Carbon to the different types of compounds it forms. The notes also provide insight into the role of Carbon in the natural environment and its impact on our daily lives. Understanding these concepts can help students develop a deeper understanding of chemistry, and its applications in various fields, such as medicine, energy, and technology. Therefore, it is crucial to take the time to review and study the Notes of Carbon and Its Compounds Class 10 thoroughly. By doing so, students can acquire a strong foundation in chemistry, which will prove valuable in their academic and professional pursuits.
Download the eSaral App for complete Class 10 Video lectures, Study material, revision and much more.
Notes of Carbon and Its Compounds Class 10
