Periodic Classification Of Elements Class 10 Notes
Class 10
Periodic Classification Of Elements Class 10 Notes explains"The periodic table displays the elements in a tabular manner, grouping elements with similar properties in the same vertical column or group."
Dobereiner's Triads and Newland's law of octaves were earlier attempts at classifying elements.
Dobereiner’s Triads: Dobereiner classified elements based on their atomic mass. He arranged elements in increasing order of atomic mass and observed that groups of three elements with similar properties were obtained. The middle element of each triad had an atomic mass that was approximately equal to the average of the atomic masses of the other two elements. For instance, Li (6.9), Na (23), and K (39) form a triad.
Limitation: It fails to arrange all the known elements in the form of triads, even having similar properties.
Newland’s Law of Octaves: This principle states that when arranging elements in order of increasing atomic masses, every eighth element displays physical and chemical properties that repeat those of the first element.
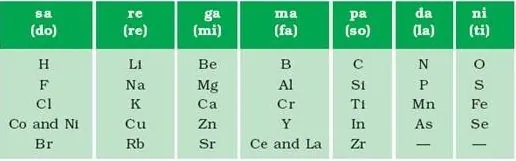
Limitations: The law of octaves was only applicable to lighter elements and was limited to calcium. Newland adjusted elements with different properties, such as Co and Ni, in the same slot with Fluorine, Chlorine, Bromine, and Iodine. He believed that only 56 elements existed in nature and that no further elements would be discovered in the future. Currently, attempts at classifying elements include Mendeleev's Periodic Table and the Modern Periodic Table.
Mendeleev’s Periodic Table: Mendeleev’s periodic table is based on the physical and chemical properties of elements and their atomic masses.
Mendeleev’s Periodic Law: According to this “The physical and chemical properties of the elements are the periodic function of their atomic masses.”
Periodicity of Properties: The repetition of properties of elements after certain regular intervals is known as Periodicity of Properties.
Merits of Mendeleev’s Periodic Table
Mendeleev's table included vacant spaces that provided an indication for discovering new elements, such as Eka-boron, Eka-aluminium, and Eka-silicon. Mendeleev's periodic table predicted the properties of several undiscovered elements based on their position in the table. Additionally, it was helpful in correcting the doubtful atomic masses of some elements. When noble gases were discovered, they could be accommodated in Mendeleev's periodic table without disrupting its order.
Limitations of Mendeleev’s Periodic Table
(1) No fixed position for hydrogen: No correct position of the hydrogen atom was in Mendeleev’s periodic table.
Example: Position of hydrogen with alkali metals and halogens (17th group).
(2) No place for isotopes: Position of isotopes were not decided.
Example: Cl-35 and Cl-37.
(3) No regular trend in atomic mass: Position of some elements with lower atomic masses before with higher atomic mass.
Example: Ni-58.7 before Co-58.9
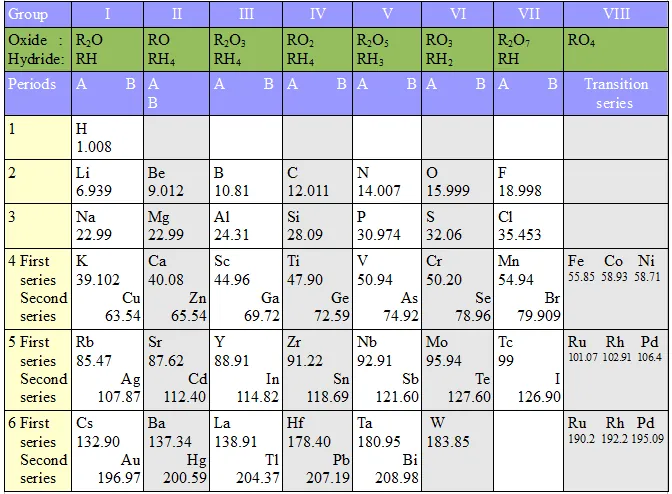
The Modern Periodic Table: In 1913, Henry Moseley showed that the atomic number of an element is a more fundamental property than its atomic mass.

Modern Period Law: The physical and chemical properties of elements follow a periodic function based on their atomic number. The modern periodic table is based on the atomic number of elements, which is equal to the number of protons present in the nucleus of an atom of an element. The table comprises 18 vertical columns called groups and seven horizontal rows known as periods. When moving from left to right in a period, the number of valence electrons increases from 1 to 8 in the elements present, while the number of shells remains the same. Additionally, all the elements in a group of the periodic table have the same number of valence electrons.
Trends in Modern Periodic Table
Valency, Atomic size, metallic and non-metallic characters, and Electronegativity.
(i) The number of valence electrons present in the outermost shell of an atom determines the valency of an element, which refers to its combining capacity.
In a period, the valency first increases from 1 to 4 and then decreases to zero (0) when moving from left to right.
In Groups: On moving from top to bottom in a group, the valency remains same because the number of valence electrons remains the same.
Example: Valency of first group elements = 1 Valency of second group elements = 2.
(ii) Atomic size:
The radius of an atom is what atomic size refers to. An isolated atom's outermost shell's distance from the nucleus's center characterizes it.
As one moves from left to right in a period, the atomic size decreases because the nuclear charge increases. For instance, in the second period, the size of elements follows this order: Li > Be > B > C > N > O > F.
It's worth noting that the atomic size of noble gases in the corresponding period is the largest due to the presence of a fully filled electronic configuration, i.e., a complete octet.
On the other hand, the atomic size increases down the group since new shells are added, regardless of the increase in nuclear charge. For example, the atomic size of elements in the first group follows this order: Li < Na < K < Rb < Cs < Fr. Also, the atomic size of elements in the 17th group follows this order: F < Cl < Br < I.
(iii) Metallic character:
Periodic Classification Of Elements Class 10 Notes -An atom's tendency to lose electrons is what we refer to as its metallic character.
As one moves from left to right along the period, the metallic character decreases because the tendency to lose electrons decreases due to the increase in nuclear charge. For example, in the second period, the metallic character follows this order: Li > Be > B > C >> N > O > F.
When moving from top to bottom in a group, the metallic character increases because both the atomic size and the tendency to lose electrons increase. For instance, the metallic character of elements in the first group follows this order: Li < Na < K < Rb < Cs. Additionally, the metallic character of elements in the 17th group follows this order: F < Cl < Br < I.
(iv) Non-metallic character:
The tendency of an atom to gain electrons is what we refer to as its non-metallic character.
As one moves from left to right along the period, the non-metallic character increases because the tendency to gain electrons increases due to the increase in nucleus charge. For example, in the second period, the non-metallic character follows this order: Li < Be < B < C < N < O < F.
On the other hand, when moving from top to bottom in a group, the non-metallic character decreases because the atomic size increases, and the tendency to gain electrons decreases. For instance, the non-metallic character of elements in the 17th period follows this order: F > Cl > Br > I.
(v) Chemical Reactivity
In metals: Chemical reactivity of metals increases down the group because tendency to lose electrons increases. Example ; Li < Na < K < Rb < Cs (1st group) In non-metals: Chemical reactivity of non-metals decreases down the group because tendency to gain electrons decreases. Example: F > Cl > Br > I (17th group)
(vi) Electronegativity:
Periodic Classification Of Elements Class 10 Notes -The tendency of an element to attract the shared pair of electrons towards it in a covalently bonded molecule is known as its electronegativity.
The electronegativity increases with an increase in nuclear charge or a decrease in atomic size.
As one moves along the period, the electronegativity increases. For instance, in the second period, the electronegativity follows this order: Li < Be < B < C < N < O < F.
On the other hand, as one moves down the group, the electronegativity decreases. For example, the electronegativity of elements in the first group follows this order: Li > Na > K > Rb > Cs. Also, the electronegativity of elements in the 17th group follows this order: F > Cl > Br > I.
(vii) Nature of Oxides: Metal oxides are basic in nature. Ex. Na2O, MgO etc.
Non-metal oxides are acidic in nature. Ex. Cl2O7, SO3, P2O5,
In the case of metal reactivity, it increases down the group because of the tendency to lose electrons increases.
In the case of non-metal reactivity, decreases down the group because of the tendency to gain electrons decreases.
Group: The vertical columns in Mendeleev’s, as well as in Modern Periodic Table, are called groups.
Period: The horizontal rows in the Modern Periodic Table and Mendeleev’s Periodic Table are called periods.
There are 18 groups and 7 (seven) periods in the Modern Periodic Table.
Atomic size: The atomic size may be visualised as the distance between the centre of the nucleus and the outermost shell of an isolated atom.
The trend of atomic size (radius) in moving down a group: As one goes down a group in the Periodic Table, the atomic size increases because atoms at every step add a new shell of electrons. This results in an increase in the distance between the outermost shell electrons and the nucleus of the atom.
The trend of atomic size (radius) in moving from left to right in a period:
As one moves from left to right along a period, the size of atoms decreases. This happens because, as we move from left to right, the atomic number of elements increases, which in turn means that the number of protons and electrons in the atoms increases.
Due to the large positive charge on the nucleus, the electrons are pulled more closely to the nucleus, resulting in a decrease in the size of the atom.
Characteristics of triads of J.W. Dobereiner.
- Elements of a triad show similar chemical properties.
- These elements of a triad show specific trends in their physical properties.
- The atomic mass of the middle element was roughly the average of the atomic masses of the other two elements.
Example: Atomic mass of Na is 23 in the triad Li, Na and K. This atomic mass is the average of the atomic masses of Li and K which have atomic masses 7 and 39 respectively.
Triads as formed by Dobereiner.
1st Triad
Li – Lithium
Na – Sodium
K – Potassium
2nd Triad
Ca – Calcium
Sr – Strontium
Ba – Barium
3rd Triad
Cl – Chlorine
Br – Bromine
I – Iodine
Mendeleev’s Periodic Law: It states that “the properties of elements are the periodic functions of their atomic masses.” It means the properties of the elements depend on their atomic masses and the elements are given a position in the periodic table on the basis of their increasing atomic masses.
Merits of Mendeleev’s Periodic Table
(i) Mendeleev left a number of gaps in his table to accommodate the new elements which would be discovered later on. So Mendeleev boldly predicted the existence of some more elements. He even predicted the properties of some of these elements and named them as Eka-boron, Eka-aluminium and Eka-silicon respectively. Later on the elements were discovered, for example, gallium replaced Eka-aluminium and it showed properties similar to that of aluminium.
(ii) He gave the proper position to the noble gases which were discovered later on, without disturbing the existing order of elements. He placed them in a new group.
Limitations of Mendeleev’s classification:
- The position of isotopes could not be explained because isotopes have the same chemical properties but different atomic masses. If the elements are arranged according to atomic masses, the isotopes should be placed in different groups of the Periodic Table.
- The atomic masses do not increase in a regular manner in going from one element to the next.
- He could not assign a correct position to hydrogen in his table because hydrogen has some properties similar to alkali metals and some properties similar to halogens.
Modern Periodic Law: This law was proposed by Henry Moseley, a scientist in 1913. According to this Law, “Properties of elements are the periodic function of their atomic number.” It means that the properties of elements depend on their atomic number and the elements are given positions in the periodic table on the basis of their increasing atomic number. As atomic number determines the distribution of electrons in the orbits, and electrons of the outermost orbit determine the properties of an element.
Groups and periods in the Modem (long form) Periodic Table: There are 18 groups (vertical columns) and 7 periods (horizontal lines) in the Modern (or long form) Periodic Table. The number of the period is equal to the number of shells in the atoms of the elements belonging to that period.
Trends in Mendeleev’s Periodic Table
- The properties of elements are periodic functions of their atomic mass.
- It has 8 groups.
- No place could be assigned to isotopes of an element.
- There were three gaps left by Mendeleev in his Periodic Table.
- No fixed position was given to hydrogen in this Periodic Table.
- No distinction was made between metals and non-metals.
- Transition elements are placed together in Group VIII.
- Inert gases were not known at the time of Mendeleev.
Trends in Modem Periodic Table - Periodic Classification Of Elements Class 10 Notes
(i) Valency: Elements belonging to the same group have the same number of valence electrons and thus the same valency. Valency in a particular period from left to right first increases as positive valency and then decreases as negative valency.
Example: In elements of 2nd period:
Li has 1+ valency, then Be2+, B3+, C4+ covalency, N3- valency, then O2- and F(-) valency.
(ii) The atomic size or atomic radius increases: as we move down in a group and it decreases as we move from left to right in a period. Atomic size increases down a group due to the increase in the number of shells. Atomic size decreases along a period due to an increase in the nuclear charge which tends to pull the electrons closer to the nucleus and reduces the size of the atom.
(iii) Metallic and Non-Metallic properties: In the modern periodic table metals are on the left side and non-metals on the right side of the table. A zig-zag line of metalloids separates metals from non-metals.
- Metallic characters decrease from left to right in a period and increase while going down in a group.
- Non-metallic characters increase from left to right in a period due to increase in the electronegativity and these characters decrease from top to bottom in a group due to the decrease in the electronegativity of atoms while going down in a group.
1. Need for classification of elements:
Increase in the discovery of different elements made it difficult to organise all that was known about the elements. To study a large number of elements with ease, various attempts were made. The attempts resulted in the classification of elements into metals and non-metals.
2. Dobereiner’s triads:
Johann Wolfgang Dobereiner, a German chemist, classified the known elements in groups of three elements on the basis of similarities in their properties. These groups were called triads.
(i) Characteristics of Triads:
- Properties of elements in each triad were similar.
- Atomic mass of the middle element was roughly the average of the atomic masses of the other two elements.
(iii) Limitations: Dobereiner could identify only three triads. He was not able to prepare triads of all the known elements.
3. Newlands’ Law of Octaves:
John Newlands’, an English scientist, arranged the known elements in the order of increasing atomic masses and called it the ‘Law of Octaves’. It is known as ‘Newlands’ Law of Octaves’.
(i) Characteristics of Newlands’ Law of Octaves:
- It contained the elements from hydrogen to thorium.
- Properties of every eighth element were similar to that of the first element.
(iii) Limitations of Newlands’ law of Octaves:
- The law was applicable to elements up to calcium (Ca).
- It contained only 56 elements.
- In order to fit elements into the table, Newlands’ adjusted two elements like cobalt and nickel in die the same slot and also put some unlike elements under the same note.
4. Mendeleev’s Periodic Table: Dmitri Ivanovich – 5 ’ Mendeleev, a Russian demist, was the most important contributor to the early development of a periodic table of elements wherein the elements were arranged on the basis of their atomic mass and chemical properties.
- Characteristics of Mendeleev’s Periodic Table:
- Mendeleev arranged all the 63 known elements in increasing order of their atomic masses.
- The table contained vertical columns called ‘groups’ and horizontal rows called ‘periods’.
- The elements with similar physical and chemical properties came under the same groups.
- Mendeleev’s Periodic Law: The properties of elements are the periodic function of their atomic masses.
- Achievements of Mendeleev’s Periodic Table-: Periodic Classification Of Elements Class 10 Notes
- Through this table, it was very easy to study the physical and chemical properties of various elements.
- Mendeleev adjusted few elements with a slightly greater atomic mass before the elements with slightly lower atomic mass, so that elements with similar properties could be grouped together. For example, aluminium appeared before silicon, cobalt appeared before nickel.
- Mendeleev left some gaps in his periodic table.
He predicted the existence of some elements that had not been discovered at that time. His predictions were quite true as elements like scandium, gallium and germanium were discovered later. - The gases like helium, neon and argon, which were discovered later, were placed in a new group without disturbing the existing order.
- Limitations:
- No fixed positions were given to hydrogen in the Mendeleev’s periodic table.
- Positions of Isotopes of all elements was not certain according to Mendeleev’s periodic table.
- Atomic masses did not increase in a regular manner in going from one element to the next.
5. Modem Periodic Table: Henry Moseley, gave a new ! property of elements, ‘atomic number’ and this was I adopted as the basis of Modem Periodic Table.
(i) Modem Periodic Law: Properties of elements are a periodic function of i their atomic number.
(ii) The position of elements in Modem Periodic Table:
- The modem periodic table consists of 18 groups and 7 periods.
- Elements present in any one group have the same number of valence electrons. Also, the number of shells increases as we go down the group.
- Elements present in any one period, contain the same number of shells. Also, with increase in atomic number by one unit on moving from left to right, the valence shell electrons increases by one unit.
- Each period marks a new
Trends in the Modern Periodic Table: - Periodic Classification Of Elements Class 10 Notes
- Valency: Valency of an element is determined by the number of valence electrons present in the outermost shell of its atom.
- Valency of elements in a particular group is same.
- Valency of elements in a period first increases from one to four and then decreases to zero.
- Atomic Size: Atomic size refers to the radius of an atom.
- In a period, atomic size and radii decreases from left to right.
- In a group, atomic size and radii increases from top to bottom.
Metallic and Non-metallic Properties: -Periodic Classification Of Elements Class 10 Notes
- The tendency to lose electrons from the outermost shell of an atom, is called metallic character of an element.
- The tendency to gain electrons from the outermost shell of an atom, is called non-metallic character of an element.
Periodic Classification Of Elements Class 10 Notes
Download the eSaral App for complete Class 10 Video lectures, Study material, revision and much more.
